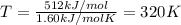Chemistry, 29.02.2020 04:18 Mistytrotter
As an approximation, we can assume that proteins exist either in their native (physiologically relevant) state or in a denatured state. The standard molar enthalpy and entropy of denaturationof a certain protein are 512 kJ/mol and 1.60 kJ/(mol K), respectively. Assume that DHand DSare independent of temperature. a. Commenton the signs thinking about protein denaturation. b. Calculate the standard molar free energy of denaturation. c. Calculate the temperature at which the denaturation becomes spontaneous.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 09:30
People who practice which of the following diets may run the risk of not getting enough iron. a. gluten free or vegan diet b. diet for managing diabetes c. vegan diet d. gluten free diet
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
As an approximation, we can assume that proteins exist either in their native (physiologically relev...
Questions


English, 09.10.2019 17:30


Mathematics, 09.10.2019 17:30

Mathematics, 09.10.2019 17:30

English, 09.10.2019 17:30

Physics, 09.10.2019 17:30

Biology, 09.10.2019 17:30

English, 09.10.2019 17:30

History, 09.10.2019 17:30


Mathematics, 09.10.2019 17:30

Computers and Technology, 09.10.2019 17:30

Social Studies, 09.10.2019 17:30


World Languages, 09.10.2019 17:30




English, 09.10.2019 17:30