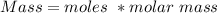Chemistry, 29.02.2020 02:54 kiarabermudez754
Calculate the weight percent of ascorbic acid in a tablet of Vitamin C from the following data:A 80 mg sample of a crushed Vitamin C tablet was dissolved in 40 mL of H2SO4 and 20 mL of water. Two grams of KI and 40. mL of 0.00653 M KIO3 solution was added, and the mixture titrated to a starch endpoint. The titration required 15 mL of 0.0510 M thiosulfate solution. I know the answer is 88%, but how did they get it?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
Calculate the weight percent of ascorbic acid in a tablet of Vitamin C from the following data:A 80...
Questions


Social Studies, 10.07.2019 10:10

Spanish, 10.07.2019 10:10

Mathematics, 10.07.2019 10:10

Health, 10.07.2019 10:10


Health, 10.07.2019 10:10

Business, 10.07.2019 10:10



Physics, 10.07.2019 10:10

Mathematics, 10.07.2019 10:10

Biology, 10.07.2019 10:10




Mathematics, 10.07.2019 10:10


English, 10.07.2019 10:10

Mathematics, 10.07.2019 10:10




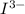 = 3 × number of moles of
= 3 × number of moles of 

 mol
mol x moles of
x moles of