
Chemistry, 29.02.2020 01:51 heyysiirr3064
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily increases, but that of O is less than that of N. The best interpretation of the lowervalue for O is that:1. the ionization potential of N is a maximum and the values decrease steadily for the elements O, F, and Ne.2. there is more shielding of the nuclear charge in O than in B, C, or N.3. the electron removed from O is farther from the nucleus and therefore less tightly bound than that in N.4. the electron removed from O corresponds to a different value of the quantum number L than that of the electron removed from B, C, or N.5. the half-filled set of p orbitals in N makes it more difficult to remove an electron from N than from O.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
The first ionization potential of the elements B, C, and N (atomic numbers 5, 6, and 7) steadily inc...
Questions


Mathematics, 07.12.2020 22:10

Mathematics, 07.12.2020 22:10

Mathematics, 07.12.2020 22:10

Computers and Technology, 07.12.2020 22:10


History, 07.12.2020 22:10

Mathematics, 07.12.2020 22:10


Mathematics, 07.12.2020 22:10



Mathematics, 07.12.2020 22:10



Biology, 07.12.2020 22:10


English, 07.12.2020 22:10

Mathematics, 07.12.2020 22:10

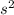 2
2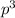 (half-filled orbital), Which allows it additional stability.
(half-filled orbital), Which allows it additional stability.


