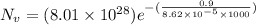Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Calculate the equilibrium number of vacancies per cubic meter for copper at 1000K. The energy for va...
Questions



History, 24.06.2021 23:10

Mathematics, 24.06.2021 23:10




Mathematics, 24.06.2021 23:10

English, 24.06.2021 23:10






Mathematics, 24.06.2021 23:10

Mathematics, 24.06.2021 23:10

Biology, 24.06.2021 23:10



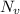 .
.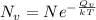
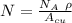
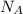 = Avogadro Number = 6.023×10²³
= Avogadro Number = 6.023×10²³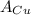 = 63.5 g/mole
= 63.5 g/mole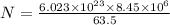
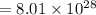 g/mole
g/mole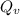 =0.9 ev/atom , T= 1000k
=0.9 ev/atom , T= 1000k