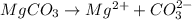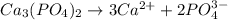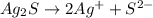Chemistry, 29.02.2020 01:24 leaving2020asap
Write the ion-product expression at equilibrium for each compound: How to enter the correct e. g. silver sulfate (Ag2SO4) enter this: {Ag+}2{SO42-} don't enter spaces, use square brackets [] not {} or () for concentration and don't worry about subscripting or superscripting [ (1) magnesium carbonate (2) calcium phosphate (3) silver sulfide

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1


Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1
You know the right answer?
Write the ion-product expression at equilibrium for each compound: How to enter the correct e. g. s...
Questions

Mathematics, 09.11.2020 19:20

History, 09.11.2020 19:20





Social Studies, 09.11.2020 19:20


French, 09.11.2020 19:20


Chemistry, 09.11.2020 19:20


Mathematics, 09.11.2020 19:20

Mathematics, 09.11.2020 19:20

Geography, 09.11.2020 19:20

History, 09.11.2020 19:20




![Q_{MgCO_3}=[Mg^{2+}][CO_3^{2-}]](/tpl/images/0528/8473/505d7.png)
![Q_{Ca_3(PO_4)_2}=[Ca^{2+}^3][PO_4^{3-}^2]](/tpl/images/0528/8473/bdee3.png)
![Q_{Ag_2S}=[Ag^{+}^2][S^{2-}]](/tpl/images/0528/8473/94d2a.png)