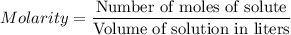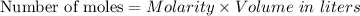Chemistry, 29.02.2020 01:25 samanthacruzsc51
For each trial, compute the mol of titrant; (molarity x L) and keep the number of significant figures to 4.
Trial 1: 12.49 mL =
mol NaOH
Trial 2: 12.32 mL =
mol NaOH
Trial 3: 11.87 mL =
mol NaOH

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 11:50
What is the oxidation half-reaction for this unbalanced redox equation? cr2o72– + fe2+ → cr3+ + fe3+ cr3+ → cr2o72– cr2o72– → cr3+ fe3+ → fe2+ fe2+ → fe3+?
Answers: 2

Chemistry, 23.06.2019 14:20
Compounds a and b react to form compounds c and d according to the equation: aa + bb → cc + dd. under which conditions will the rate law be given by the equation: rate = k[a]a[b]b? a. the reaction takes place in one step. b. the reaction is endothermic. c. the reaction is exothermic. d. the reaction involves more than one step.
Answers: 3
You know the right answer?
For each trial, compute the mol of titrant; (molarity x L) and keep the number of significant figure...
Questions

French, 25.11.2020 01:20


English, 25.11.2020 01:20

Physics, 25.11.2020 01:20


History, 25.11.2020 01:20

Chemistry, 25.11.2020 01:20


Mathematics, 25.11.2020 01:20


Mathematics, 25.11.2020 01:20


Mathematics, 25.11.2020 01:20

Health, 25.11.2020 01:20

Mathematics, 25.11.2020 01:20





Arts, 25.11.2020 01:20