
Chemistry, 28.02.2020 23:27 emobirdy1122
Consider the following multistep reaction: A+B→AB(slow) A+AB→A2B(fast)–––––––––––––––––––– 2A+B→A2B(overall) Based on this mechanism, determine the rate law for the overall reaction. Express your answer in standard MasteringChemistry format. For example, if the rate law is k[A]3[B]2 type k*[A]^3*[B]^2. View Available Hint(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
Consider the following multistep reaction: A+B→AB(slow) A+AB→A2B(fast)–––––––––––––––––––– 2A+B→A2B(...
Questions



Mathematics, 07.10.2019 15:20

Physics, 07.10.2019 15:20


Chemistry, 07.10.2019 15:20




Mathematics, 07.10.2019 15:20



History, 07.10.2019 15:20

Arts, 07.10.2019 15:20

Mathematics, 07.10.2019 15:20

Biology, 07.10.2019 15:20



Mathematics, 07.10.2019 15:20

Biology, 07.10.2019 15:20

![Rate=k[A][B]](/tpl/images/0528/6935/27e48.png)
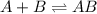 (slow)
(slow)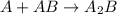 (fast)
(fast)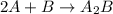 (overall)
(overall)

