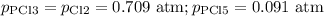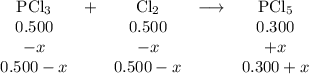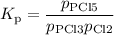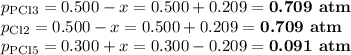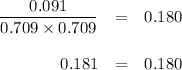Chemistry, 29.02.2020 00:17 Dogtes9667
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.
A flask is charged with 0.500 atm PCl3 , 0.500 atm Cl2, and 0.300atm PCl5 at this temperature.
What are the equilibrium partial pressures of PCl3 , Cl2, and PCl5, respectively?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1


You know the right answer?
For the exothermic reaction
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
PCl3(g)+Cl2(g)?PCl5(g)
Kp = 0.180 at a certain temperature.<...
Questions

History, 17.10.2021 14:00

English, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00




English, 17.10.2021 14:00


Mathematics, 17.10.2021 14:00

Physics, 17.10.2021 14:00

English, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00

Biology, 17.10.2021 14:00


Physics, 17.10.2021 14:00

Geography, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00


Mathematics, 17.10.2021 14:00