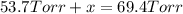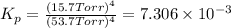Chemistry, 28.02.2020 22:46 AutumnJoy12
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressure of water vapor is 53.7 torr, the total pressure at equilibrium is 69.4 torr. Calculate Kp for this reaction at 1181 K. 3 Fe(s) + 4 H2O(g) equilibrium reaction arrow Fe3O4(s) + 4 H2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
In a study of the reaction below at 1181 K, it was observed that when the equilibrium partial pressu...
Questions

Mathematics, 01.11.2019 11:31





Biology, 01.11.2019 11:31




Mathematics, 01.11.2019 11:31


Social Studies, 01.11.2019 11:31

Mathematics, 01.11.2019 11:31



Mathematics, 01.11.2019 11:31


Mathematics, 01.11.2019 11:31

Mathematics, 01.11.2019 11:31

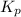 for this reaction at 1181 K is
for this reaction at 1181 K is  .
.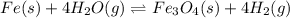
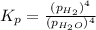
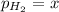
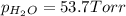
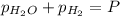 (Dalton's law of partial pressure)
(Dalton's law of partial pressure)