Chemistry, 28.02.2020 22:51 wkalpakchi
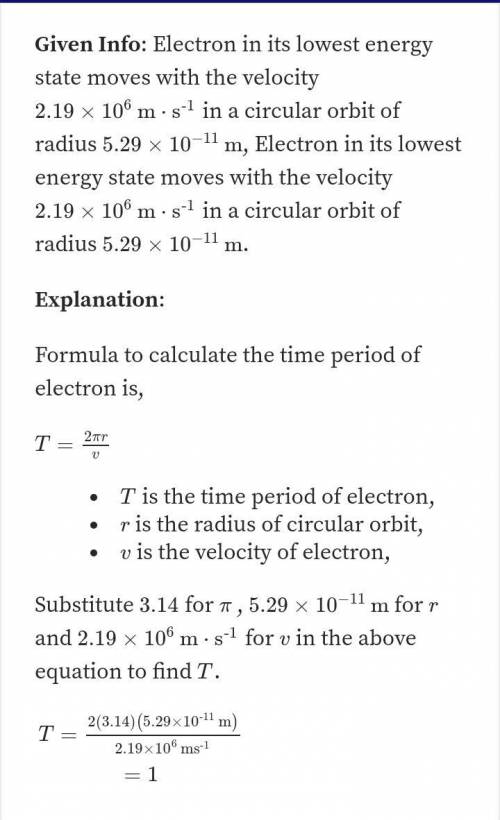
In the Bohr model of the hydrogen atom, an electron in the 8th excited state moves at a speed of 3.42 104 m/s in a circular path having a radius of 3.39 10-9 m. What is the effective current associated with this orbiting electron

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

You know the right answer?
In the Bohr model of the hydrogen atom, an electron in the 8th excited state moves at a speed of 3.4...
Questions

Mathematics, 05.05.2020 20:24


Biology, 05.05.2020 20:24

Mathematics, 05.05.2020 20:24

Mathematics, 05.05.2020 20:24

English, 05.05.2020 20:24





Mathematics, 05.05.2020 20:24



Mathematics, 05.05.2020 20:24





Biology, 05.05.2020 20:24