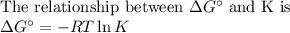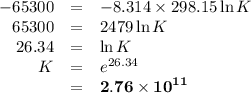Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
Sing any data you can find in the ALEKS Data resource, calculate the equilibrium constant K at 25.0°...
Questions

Mathematics, 06.10.2019 07:40


Social Studies, 06.10.2019 07:40


Mathematics, 06.10.2019 07:40



Mathematics, 06.10.2019 07:40

Geography, 06.10.2019 07:40


Chemistry, 06.10.2019 07:40









Mathematics, 06.10.2019 07:40