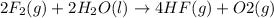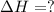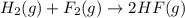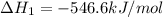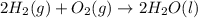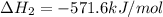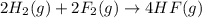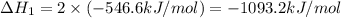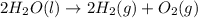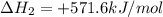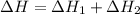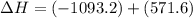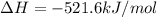Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Given that H 2 ( g ) + F 2 ( g ) ⟶ 2 HF ( g ) Δ H ∘ rxn = − 546.6 kJ ⋅ mol − 1 2 H 2 ( g ) + O 2 ( g...
Questions

Mathematics, 14.01.2021 14:00

World Languages, 14.01.2021 14:00

Social Studies, 14.01.2021 14:00

Physics, 14.01.2021 14:00

Mathematics, 14.01.2021 14:00




World Languages, 14.01.2021 14:00


Mathematics, 14.01.2021 14:00

Mathematics, 14.01.2021 14:00

Mathematics, 14.01.2021 14:00


Mathematics, 14.01.2021 14:00

Mathematics, 14.01.2021 14:00


Biology, 14.01.2021 14:00

Mathematics, 14.01.2021 14:00

Chemistry, 14.01.2021 14:00