
Chemistry, 28.08.2019 14:50 meadowsoares7
The balanced chemical equation for the reaction of copper (cu) and silver nitrate (agno3) is shown below.
cu + 2agno3 mc001-1.jpg 2ag + cu(no3)2
how many moles of copper must react to form 3.50 mol of ag?
0.175 mol
1.75 mol
7.00 mol
17.0 mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

You know the right answer?
The balanced chemical equation for the reaction of copper (cu) and silver nitrate (agno3) is shown b...
Questions

History, 20.11.2021 14:00





Mathematics, 20.11.2021 14:00


History, 20.11.2021 14:00


Computers and Technology, 20.11.2021 14:00

Computers and Technology, 20.11.2021 14:00

Biology, 20.11.2021 14:00

Arts, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00

Mathematics, 20.11.2021 14:00





Chemistry, 20.11.2021 14:00

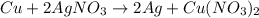
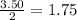 moles of copper
moles of copper

