
Chemistry, 28.02.2020 19:28 alexantkoviak13
The rate of disappearance of HBr in the gas phase reaction 2HBr(g)→H2(g)+Br2(g) is 0.360 Ms−1 at 150 ∘C. The rate of appearance of Br2 is Ms−1. The rate of disappearance of in the gas phase reaction is 0.360 at 150 . The rate of appearance of is . 1.39 0.600 0.720 0.180 0.130

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
The rate of disappearance of HBr in the gas phase reaction 2HBr(g)→H2(g)+Br2(g) is 0.360 Ms−1 at 150...
Questions


Chemistry, 21.01.2021 22:30



Mathematics, 21.01.2021 22:30

Mathematics, 21.01.2021 22:30

Mathematics, 21.01.2021 22:30







Mathematics, 21.01.2021 22:30


Mathematics, 21.01.2021 22:30

Chemistry, 21.01.2021 22:30

Mathematics, 21.01.2021 22:30

Social Studies, 21.01.2021 22:30

Physics, 21.01.2021 22:30

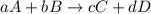
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0528/1964/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0528/1964/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0528/1964/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0528/1964/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0528/1964/d4b94.png)
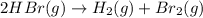
![\text{Rate of disappearance of }HBr=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0528/1964/d63dd.png)
![\text{Rate of appearance of }H_2=+\frac{d[H_2]}{dt}](/tpl/images/0528/1964/7fea8.png)
![\text{Rate of appearance of }Br_2=+\frac{d[Br_2]}{dt}](/tpl/images/0528/1964/d3d56.png)
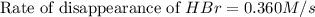
![+\frac{d[Br_2]}{dt}=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0528/1964/3e9c6.png)
![\frac{d[Br_2]}{dt}=\frac{1}{2}\times 0.360M/s](/tpl/images/0528/1964/53e73.png)
![\frac{d[Br_2]}{dt}=0.180M/s](/tpl/images/0528/1964/3fdb1.png)


