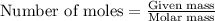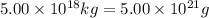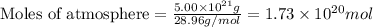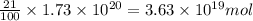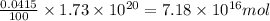The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 are present in the atmosphere? Note that it is important to work in units of moles rather than in units of mass. By the ideal gas law, PV=nRT. P is pressure, V is volume, n is the number of moles, T is temperature (K), and R is the gas constant. At a given temperature and pressure, the volume is proportional to the number of moles, not to the mass.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2


Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
The total mass of the atmosphere is about 5.00 x 1018 kg. How many moles each of air, O2, and CO2 ar...
Questions




Spanish, 13.02.2020 04:27


Mathematics, 13.02.2020 04:27

Mathematics, 13.02.2020 04:27

Physics, 13.02.2020 04:27







History, 13.02.2020 04:28


History, 13.02.2020 04:28


Biology, 13.02.2020 04:28

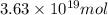 and
and 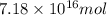 respectively
respectively