
Chemistry, 28.02.2020 19:11 sadmomsclub
Suppose the first equation is reversed and multiplied by 1/6, the second and third equations are divided by 2, and the three adjusted equations are added. What is the net reaction?3 A + 6 B → 3 D, ΔH = -402 kJ/molE + 2 F → A, ΔH = -108.3 kJ/molC → E + 3 D, ΔH = +65.5 kJ/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

You know the right answer?
Suppose the first equation is reversed and multiplied by 1/6, the second and third equations are div...
Questions

English, 10.10.2019 02:30

Mathematics, 10.10.2019 02:30



Mathematics, 10.10.2019 02:30

Mathematics, 10.10.2019 02:30

Mathematics, 10.10.2019 02:30

Mathematics, 10.10.2019 02:30

Biology, 10.10.2019 02:30

History, 10.10.2019 02:30










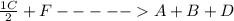 ;ΔH = + 45.6 kJ/mol
;ΔH = + 45.6 kJ/mol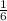 ; we have:
; we have: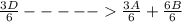 ; Δ
; Δ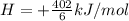
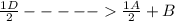 ; Δ
; Δ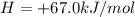 ----Equation 1
----Equation 1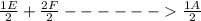 ;ΔH =
;ΔH = 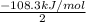
 ;ΔH = -54.15 kJ/mol --- Equation 2
;ΔH = -54.15 kJ/mol --- Equation 2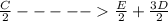 ;ΔH =
;ΔH = 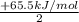
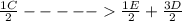 ;ΔH = + 32.75 kJ/mol --- Equation 3
;ΔH = + 32.75 kJ/mol --- Equation 3


