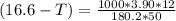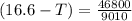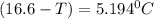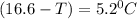Chemistry, 28.02.2020 19:15 noobgirlaskthequest
What is the freezing point of a solution that contains 12.0 g of glucose (C6H12O6) in 50 g of acetic acid (CH3COOH). For acetic acid, Kf is 3.90°C/m and the melting point is 16.6 °C

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
What is the freezing point of a solution that contains 12.0 g of glucose (C6H12O6) in 50 g of acetic...
Questions









Mathematics, 29.04.2021 21:40

Social Studies, 29.04.2021 21:40


Chemistry, 29.04.2021 21:40



Mathematics, 29.04.2021 21:40

English, 29.04.2021 21:40

Computers and Technology, 29.04.2021 21:40


Mathematics, 29.04.2021 21:40