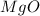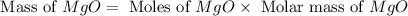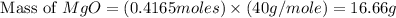Chemistry, 28.02.2020 19:57 stinematesa
You may want to reference (Page) section 4.3 while completing this problem. Magnesium oxide can be made by heating magnesium metal in the presence of the oxygen. The balanced equation for the reaction is 2Mg(s)+O2(g)→2MgO(s) 2 M g ( s ) + O 2 ( g ) → 2 M g O ( s ) When 10.0 g g Mg M g is allowed to react with 10.6 g g O2 O 2 , 12.1 g g MgO Mg O is collected.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
You may want to reference (Page) section 4.3 while completing this problem. Magnesium oxide can be m...
Questions




English, 18.07.2019 12:00


Mathematics, 18.07.2019 12:00


Social Studies, 18.07.2019 12:00

Biology, 18.07.2019 12:00

Computers and Technology, 18.07.2019 12:00





Health, 18.07.2019 12:00



Mathematics, 18.07.2019 12:00

Biology, 18.07.2019 12:00

Health, 18.07.2019 12:00

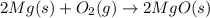
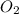 = 10.6 g
= 10.6 g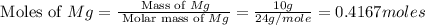
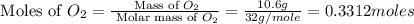
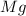 react with 1 mole of
react with 1 mole of 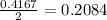 moles of
moles of