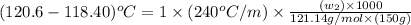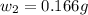A certain liquid X has a normal boiling point of 118.40 C and a boiling point elevation constant K,-240 С kg-mol-1. A solution is prepared by dissolving some benzamide (C, H,NO) in 150. g of X. This solution boils at 120.6 C. Calculate the mass of C, H,NO that was dissolved Be sure your answer is rounded to the correct number of significiant digits.

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1

Chemistry, 23.06.2019 09:40
Write balanced nuclear equations for the formation of five elements whose atomic number is between helium (2) and iron (26):
Answers: 1
You know the right answer?
A certain liquid X has a normal boiling point of 118.40 C and a boiling point elevation constant K,-...
Questions

Social Studies, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40

Physics, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40


Social Studies, 17.11.2020 17:40


Advanced Placement (AP), 17.11.2020 17:40

Social Studies, 17.11.2020 17:40


Mathematics, 17.11.2020 17:40

Mathematics, 17.11.2020 17:40

Biology, 17.11.2020 17:40


English, 17.11.2020 17:40

English, 17.11.2020 17:40




Mathematics, 17.11.2020 17:40

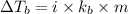
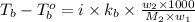
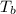 = boiling point of solution =
= boiling point of solution = 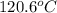
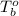 = boiling point of liquid X =
= boiling point of liquid X = 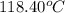
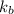 = boiling point constant of liquid X =
= boiling point constant of liquid X = 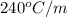
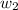 = mass of solute (benzamide ) = ?
= mass of solute (benzamide ) = ?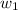 = mass of solvent (liquid X) = 150 g
= mass of solvent (liquid X) = 150 g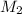 = molar mass of solute (benzamide ) = 121.14 g/mol
= molar mass of solute (benzamide ) = 121.14 g/mol