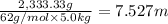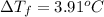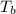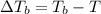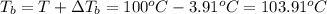Some ethylene glycol, , is added to your car’s cooling system along with 5.0 kg of water.
a. If the freezing point of the water–glycol solution is −14.0 °C, what mass of must have been added?
b. What is the boiling point of the coolant mixture? Kb(H20) = 0.52 degrees celcius kg mol^-1.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Some ethylene glycol, , is added to your car’s cooling system along with 5.0 kg of water.
Questions

History, 03.11.2020 03:20


English, 03.11.2020 03:20

Mathematics, 03.11.2020 03:20

Chemistry, 03.11.2020 03:20

Mathematics, 03.11.2020 03:20


Chemistry, 03.11.2020 03:20

History, 03.11.2020 03:20


Mathematics, 03.11.2020 03:20



Health, 03.11.2020 03:20


Chemistry, 03.11.2020 03:20



History, 03.11.2020 03:20

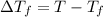
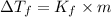
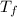 = freezing point of solution
= freezing point of solution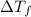 =depression in freezing point
=depression in freezing point 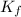 = freezing point constant
= freezing point constant 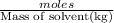
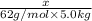
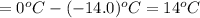
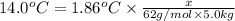
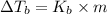
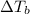 =elevation in boiling point =
=elevation in boiling point = 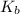 = boiling point constant
= boiling point constant