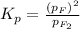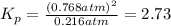At 500 degree C, F_2 gas is stable and does not dissociate, but at 840 degree C, some dissociation occurs: F_2 (g) 2 F(g). A flask filled with 0.600 atm of F_2 at 500 degree C was heated to 840 degree C, and the pressure at equilibrium was measured to be 0.984 atm. What is the equilibrium constant K_p for the dissociation of F_2 gas at 840 degree C?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
At 500 degree C, F_2 gas is stable and does not dissociate, but at 840 degree C, some dissociation o...
Questions

Arts, 08.10.2020 08:01



Geography, 08.10.2020 08:01


Physics, 08.10.2020 08:01


Mathematics, 08.10.2020 08:01


History, 08.10.2020 08:01


Mathematics, 08.10.2020 08:01

Biology, 08.10.2020 08:01

Biology, 08.10.2020 08:01

Computers and Technology, 08.10.2020 08:01

Social Studies, 08.10.2020 08:01

Biology, 08.10.2020 08:01



Mathematics, 08.10.2020 08:01

 gas at 840 degree Celsius.
gas at 840 degree Celsius.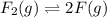
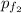 = (0.600 atm - 0.384 atm)=0.216 atm
= (0.600 atm - 0.384 atm)=0.216 atm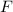 gas,
gas, 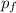 = 2(0.384 atm)=0.768 atm
= 2(0.384 atm)=0.768 atm