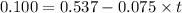Chemistry, 28.02.2020 02:25 kittykat7315
The rate constant for a particular zero-order reaction is 0.075 M s^-1. If the initial concentration of reactant is 0.537 M it takes s for the concentration to decrease to 0.100 M. A) 5.8 B) -5.8 C) -0.047 D) 7.2 E) 0.040

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3
You know the right answer?
The rate constant for a particular zero-order reaction is 0.075 M s^-1. If the initial concentration...
Questions


Mathematics, 31.10.2020 01:00


Mathematics, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00


Mathematics, 31.10.2020 01:00

Business, 31.10.2020 01:00


History, 31.10.2020 01:00




Mathematics, 31.10.2020 01:00



English, 31.10.2020 01:00

Biology, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00

Chemistry, 31.10.2020 01:00

![[A_t] = [A_0] -kt](/tpl/images/0527/6181/5739c.png)
![[A_t]](/tpl/images/0527/6181/5262c.png) is the final concentration = 0.100 M
is the final concentration = 0.100 M![[A_0]](/tpl/images/0527/6181/9a686.png) is the initial concentration = 0.537 M
is the initial concentration = 0.537 M