
Chemistry, 28.02.2020 02:23 melissapulido198
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-2 at 566 K .The reaction is allowed to reach equilibrium in a 7.40-L flask. At equilibrium, [SbCl5] = 0.333 M, [SbCl3] = 8.75×10-2 M and [Cl2] = 8.75×10-2 M.
(a) The equilibrium mixture is transferred to a 14.8-L flask. In which direction will the reaction proceed to reach equilibrium?
(b) Calculate the new equilibrium concentrations that result when the equilibrium mixture is transferred to a 14.8-L flask.
[SbCl5] = M
[SbCl3] = M
[Cl2] = M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
Consider the equilibrium between SbCl5, SbCl3 and Cl2. SbCl5(g) -->SbCl3(g) + Cl2(g) K = 2.30×10-...
Questions

Mathematics, 18.12.2020 14:00

Social Studies, 18.12.2020 14:00



English, 18.12.2020 14:00


English, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00

SAT, 18.12.2020 14:00

Mathematics, 18.12.2020 14:00


Mathematics, 18.12.2020 14:00




Arts, 18.12.2020 14:00


Mathematics, 18.12.2020 14:00


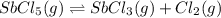
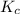
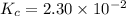
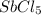 in 7.40 L = 0.333 M
in 7.40 L = 0.333 M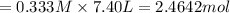
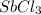 in 7.40 L =
in 7.40 L = 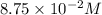
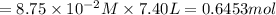
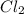 in 7.40 L =
in 7.40 L = 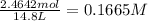
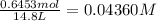
![K_c=\frac{[SbCl_3][Cl_2]}{[SbCl_5]}](/tpl/images/0527/6048/c5c78.png)
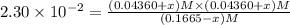
![[SbCl_5]=(0.1665-x) M=(0.1665-0.01536) M=0.1511 M](/tpl/images/0527/6048/35e3f.png)
![[SbCl_3]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/a8c15.png)
![[Cl_2]=(0.04360+x) M=(0.04360+0.01536) M=0.05896 M](/tpl/images/0527/6048/dab14.png)


