
Chemistry, 27.02.2020 23:53 hoytkeke6776
A solution with a total volume of 1000.0 mL contains 37.1 g Mg(NO3)2. If you remove 20.0 mL of this solution and then dilute this 20.0 mL sample with water until the new volume equals 500.0 mL, what is the concentration of Mg 2 ion in the 500.0 mL of solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
You know the right answer?
A solution with a total volume of 1000.0 mL contains 37.1 g Mg(NO3)2. If you remove 20.0 mL of this...
Questions

Social Studies, 13.02.2021 03:30

English, 13.02.2021 03:30

English, 13.02.2021 03:30


English, 13.02.2021 03:30

Mathematics, 13.02.2021 03:30

Social Studies, 13.02.2021 03:30




Computers and Technology, 13.02.2021 03:30

Mathematics, 13.02.2021 03:30

Mathematics, 13.02.2021 03:30

Mathematics, 13.02.2021 03:30


Mathematics, 13.02.2021 03:30


Mathematics, 13.02.2021 03:30

Geography, 13.02.2021 03:30

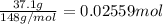
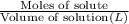
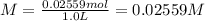
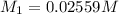
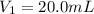
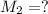
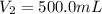
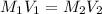 (dilution )
(dilution )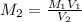
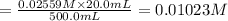
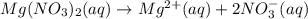
![[Mg^{2+}]=1\times 0.01023 M=0.01023 M](/tpl/images/0527/3801/31d0c.png)


