
Chemistry, 27.02.2020 22:14 epmooneyham922
In part 3 of the lab, you discovered the relationship between temperature (T) and volume (V) of a gas.
Write a mathematical equation showing that volume (V) divided by temperature (T) remains equal to a constant (k).
Hint: Use the forward slash (/) as the divided-by sign and remember to use proper capitalization.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Initially, the balloon had 3.0 liters of gas at a pressure of 400 kpa and was at a temperature of 294 k. if the balloon is cooled to 277 k and its volume decreased to 1 l, what will the new pressure in the balloon be?
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
In part 3 of the lab, you discovered the relationship between temperature (T) and volume (V) of a ga...
Questions



Computers and Technology, 29.02.2020 00:26




Mathematics, 29.02.2020 00:26







Biology, 29.02.2020 00:26




Mathematics, 29.02.2020 00:26


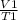 = k
= k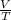 = k
= k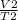 =
= 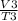 ...
...

