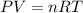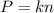Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g of Ne, and the other flask contains 10.0 g of He. Is each of the following statements true or false? Explain.1) True. Because the gases have the same mass, they exert the same pressures.2) True. Both the volumes and the temperatures of the gases are the same, which means that their pressures must be equal.3) False. There are different numbers of moles in the flasks, meaning that the pressures are different.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Two flasks of equal volume and at the same temperature contain different gases. One flask contains 1...
Questions

Mathematics, 22.06.2019 19:00


Biology, 22.06.2019 19:00

Chemistry, 22.06.2019 19:00

Biology, 22.06.2019 19:00


Mathematics, 22.06.2019 19:00


Mathematics, 22.06.2019 19:00


Mathematics, 22.06.2019 19:00

Mathematics, 22.06.2019 19:00

History, 22.06.2019 19:00

History, 22.06.2019 19:00

English, 22.06.2019 19:00




Mathematics, 22.06.2019 19:00