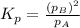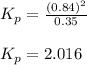Chemistry, 27.02.2020 19:50 superfly903
Consider the hypothetical reaction A(g)←→2B(g). A flask is charged with 0.77 atm of pure A, after which it is allowed to reach equilibrium at 0 ∘C. At equilibrium the partial pressure of A is 0.35 atm .A: What is the total pressure in the flask at equilibrium?
B:What is the value of Kp?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 00:30
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Consider the hypothetical reaction A(g)←→2B(g). A flask is charged with 0.77 atm of pure A, after wh...
Questions









Biology, 16.04.2020 16:22



Mathematics, 16.04.2020 16:22







Chemistry, 16.04.2020 16:22

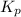 for the given equation is 2.016
for the given equation is 2.016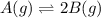
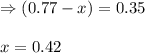
![p^A_{eq}+p^b_{eq}=[0.35+0.84]atm=1.19atm](/tpl/images/0527/0172/9c43a.png)