
Be sure to answer all parts. Consider the following equilibrium process at 686°C: CO2(g) + H2(g) ⇌ CO(g) + H2O(g) The equilibrium concentrations of the reacting species are [CO] = 0.0500 M, [H2] = 0.0410 M, [CO2] = 0.0880 M, and [H2O] = 0.0380 M. (a) Calculate Kc for the reaction at 686°C.(b) If we add CO2 to increase its concentrationto 0.30 mol/L, what will theconcentrations of all the gases be when equilibrium isreestablished?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 09:30
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3

Chemistry, 23.06.2019 17:10
What can form as a result of a chemical reaction? what can form as a result of a chemical reaction? compounds isotopes alpha particles beta particles
Answers: 2
You know the right answer?
Be sure to answer all parts. Consider the following equilibrium process at 686°C: CO2(g) + H2(g) ⇌ C...
Questions

Mathematics, 10.02.2020 18:38







Mathematics, 10.02.2020 18:39




History, 10.02.2020 18:39

Mathematics, 10.02.2020 18:39

English, 10.02.2020 18:39


Mathematics, 10.02.2020 18:40




![[CO_2]=0.2834 M](/tpl/images/0526/9800/c4d54.png)
![[H_2]=0.02440 M](/tpl/images/0526/9800/4ccc4.png)
![[CO]=0.06660 M](/tpl/images/0526/9800/7fe11.png)
![[H_2O]=0.05460 M](/tpl/images/0526/9800/56dcb.png)
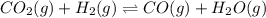
![[CO]=0.0500 M](/tpl/images/0526/9800/16f57.png)
![[H_2]=0.0410 M](/tpl/images/0526/9800/8d6da.png)
![[CO_2]=0.0880 M](/tpl/images/0526/9800/fdfdd.png)
![[H_2O]=0.0380 M](/tpl/images/0526/9800/4cb1c.png)
![K_c=\frac{[CO][H_2O]}{[CO_2][H_2]}](/tpl/images/0526/9800/c597d.png)
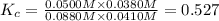
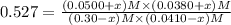
![[CO_2]=(0.30-x)=(0.30-0.0166) M=0.2834 M](/tpl/images/0526/9800/94c3e.png)
![[H_2]=(0.0410-x)=(0.0410-0.0166) M=0.02440 M](/tpl/images/0526/9800/e30dc.png)
![[CO]=(0.0500+x)=(0.0500+0.0166) M=0.06660 M](/tpl/images/0526/9800/af1c7.png)
![[H_2O]=(0.0380+x)=(0.0380+0.0166) M=0.05460 M](/tpl/images/0526/9800/b2417.png)


