
Chemistry, 27.02.2020 18:30 pineapplefun
Immunoglobulin G(IgG), formerly called gamma globulin, is a principle antibody in blood serum. A 0.457 gram sample of immunoglobulin G is dissolved in water to make 0.123 L of solution, and the osmotic pressure of the solution at 25C is found to be 0.614 mbar. Calculate the molecular mass of immunoglobulin G.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
Immunoglobulin G(IgG), formerly called gamma globulin, is a principle antibody in blood serum. A 0.4...
Questions

Biology, 28.04.2021 16:50


Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Mathematics, 28.04.2021 16:50

Social Studies, 28.04.2021 16:50

History, 28.04.2021 16:50


Biology, 28.04.2021 17:00


Mathematics, 28.04.2021 17:00

Mathematics, 28.04.2021 17:00

Mathematics, 28.04.2021 17:00

Mathematics, 28.04.2021 17:00



Computers and Technology, 28.04.2021 17:00

Mathematics, 28.04.2021 17:00

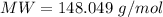 where MW is molar mass
where MW is molar mass



