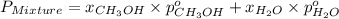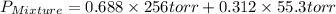Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
A solution of methanol and water has a mole fraction of water of 0.312 and a total vapor pressure of...
Questions


Mathematics, 26.10.2020 03:40


Mathematics, 26.10.2020 03:40

Mathematics, 26.10.2020 03:40

Mathematics, 26.10.2020 03:40


Mathematics, 26.10.2020 03:40

Mathematics, 26.10.2020 03:40

Mathematics, 26.10.2020 03:40




History, 26.10.2020 03:40

Mathematics, 26.10.2020 03:40


History, 26.10.2020 03:40

Mathematics, 26.10.2020 03:40

Mathematics, 26.10.2020 03:40

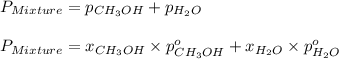
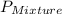 = total vapor pressure of mixture
= total vapor pressure of mixture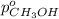 = vapor pressure of pure methanol = 256 torr
= vapor pressure of pure methanol = 256 torr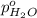 = vapor pressure of pure water = 55.3 torr
= vapor pressure of pure water = 55.3 torr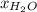 = mole fraction of water = 0.312
= mole fraction of water = 0.312 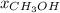 = mole fraction of methanol = 1 - 0.312 = 0.688
= mole fraction of methanol = 1 - 0.312 = 0.688