Chemistry, 27.02.2020 09:25 garacey241
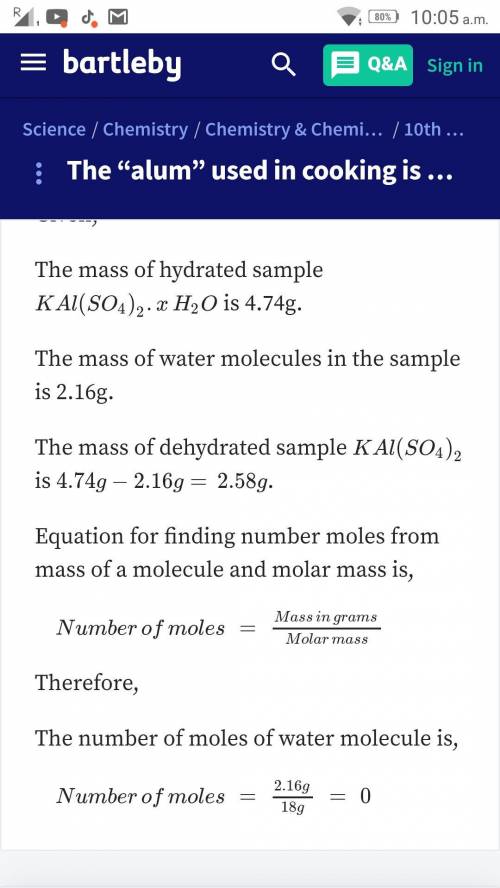
Alum used in cooking is potassium aluminum sulfate hydrate, KAl(SO4)2. XH2O. To find the value of X, you can heat the sample of the compound. Assume you heat 4.74 g of the hydrated compound and that sample loses 2.16 g of water. What is the value of X?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Alum used in cooking is potassium aluminum sulfate hydrate, KAl(SO4)2. XH2O. To find the value of X,...
Questions

Advanced Placement (AP), 10.12.2020 19:50



Mathematics, 10.12.2020 19:50


Mathematics, 10.12.2020 19:50



Social Studies, 10.12.2020 19:50


Mathematics, 10.12.2020 19:50

Mathematics, 10.12.2020 19:50



Mathematics, 10.12.2020 19:50

Mathematics, 10.12.2020 19:50


Chemistry, 10.12.2020 19:50


Mathematics, 10.12.2020 19:50