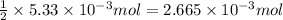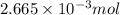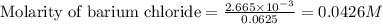A 20.00 mL Ba ( OH ) 2 solution of unknown concentration was neutralized by the addition of 42.50 mL of a 0.1255 M HCl solution. Write the balanced molecular equation for the neutralization reaction between HCl and Ba ( OH ) 2 in aqueous solution. Include physical states. molecular equation: Ba^{2+}(aq) +2OH^{-}(aq) +H^{+}(aq) +Cl^{-}(aq)<=>H_{2}O(l) +Ba^{2+}(aq) +Cl^{-}(aq) Ba 2 + ( aq ) + 2 OH − ( aq ) + H + ( aq ) + Cl − ( aq ) − ⇀ ↽ − H 2 O ( l ) + Ba 2 + ( aq ) + Cl − ( aq ) Calculate the concentration of Ba ( OH ) 2 in the original 20.00 mL solution. [ Ba ( OH ) 2 ] = M Calculate the concentrations of Ba 2 + and Cl − in solution following the neutralization reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
A 20.00 mL Ba ( OH ) 2 solution of unknown concentration was neutralized by the addition of 42.50 mL...
Questions


History, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00

French, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00


Chemistry, 05.05.2021 14:00

History, 05.05.2021 14:00



Mathematics, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00

Chemistry, 05.05.2021 14:00

English, 05.05.2021 14:00



Mathematics, 05.05.2021 14:00

History, 05.05.2021 14:00

Mathematics, 05.05.2021 14:00

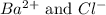 ions in the solution are 0.0426 M and 0.0852 M respectively
ions in the solution are 0.0426 M and 0.0852 M respectively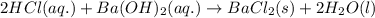
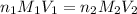
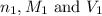 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl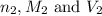 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is 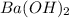
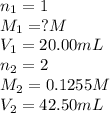
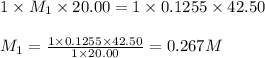
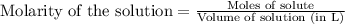 .....(1)
.....(1)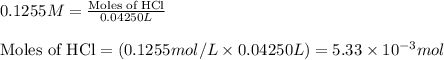
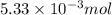 of HCl will produce =
of HCl will produce =