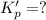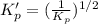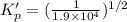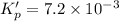Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
For the following reaction, Kp = 1.9 ✕ 104 at 1722 K. H2(g) + Br2(g) equilibrium reaction arrow 2 HB...
Questions

Biology, 30.12.2019 17:31

Mathematics, 30.12.2019 17:31












Chemistry, 30.12.2019 17:31





Mathematics, 30.12.2019 17:31

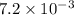
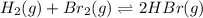 ;
; 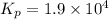
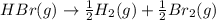 ;
;