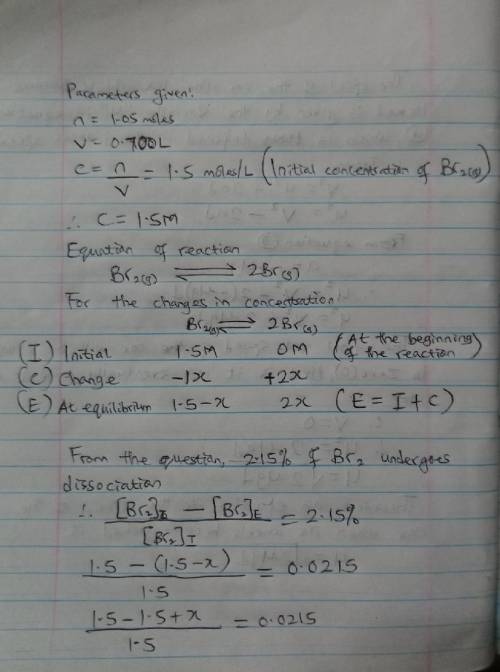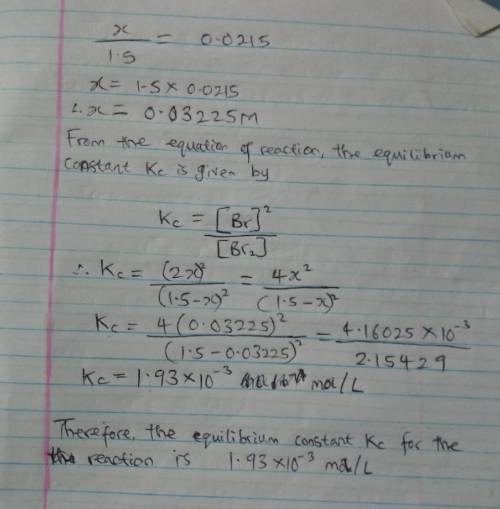Chemistry, 27.02.2020 03:11 greatsavagebeast
When 1.05 moles of Br2 are put in a 0.700−L flask, 2.15 percent of the Br2 undergoes dissociation. Calculate the equilibrium constant Kc for the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

You know the right answer?
When 1.05 moles of Br2 are put in a 0.700−L flask, 2.15 percent of the Br2 undergoes dissociation. C...
Questions






Mathematics, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

English, 18.10.2020 14:01


History, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Arts, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Computers and Technology, 18.10.2020 14:01

Arts, 18.10.2020 14:01