
Chemistry, 27.02.2020 02:02 TombRaider167
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid serious problems. One such breathing mixture contains helium, oxygen, and carbon dioxide. Determine the partial pressure of oxygen when the total pressure in the tank is 201.4 kPa if PHe = 125.4 kPa and PCO2= 18.2 kPa? Must show all work that leads to answer for credit

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Deep-sea divers must use special gas mixtures in their tanks, rather than compressed air, to avoid s...
Questions

Mathematics, 06.12.2021 19:40

Computers and Technology, 06.12.2021 19:40


Spanish, 06.12.2021 19:40






Computers and Technology, 06.12.2021 19:40


World Languages, 06.12.2021 19:40


Mathematics, 06.12.2021 19:40



Mathematics, 06.12.2021 19:40


Computers and Technology, 06.12.2021 19:40

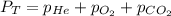
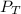 = 201.4 kPa
= 201.4 kPa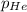 = 125.4 kPa
= 125.4 kPa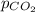 = 18.2 kPa
= 18.2 kPa![201.4=125.4+p_{O_2}+18.2\\\\p_{O_2}=201.4-[125.4+18.2]=57.8kPa](/tpl/images/0526/1098/fa869.png)


