
Chemistry, 27.02.2020 01:24 realpcy7515
The student is now told that the four solids, in no particular order, are aluminum chloride (AlCl3AlCl3), sugar (C6H12O6C6H12O6), benzoic acid (C6H5COOHC6H5COOH), and sodium bromide (NaBrNaBr). Assuming that conductivity is correlated to the number of ions in solution, rank the four substances based on how well a 0.20 MM solution in water will conduct electricity.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1


Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
The student is now told that the four solids, in no particular order, are aluminum chloride (AlCl3Al...
Questions




Biology, 19.01.2021 01:00

Mathematics, 19.01.2021 01:00



Mathematics, 19.01.2021 01:00


Mathematics, 19.01.2021 01:00

Social Studies, 19.01.2021 01:00

Mathematics, 19.01.2021 01:00



Arts, 19.01.2021 01:00

Physics, 19.01.2021 01:00

Mathematics, 19.01.2021 01:00



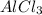 > NaBr > Benzoic acid > sugar
> NaBr > Benzoic acid > sugar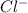 ions and thus, produces 4 ions.
ions and thus, produces 4 ions.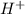 and
and 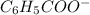 .
.

