
Chemistry, 27.02.2020 00:58 aredwolf2017
Enter your answer in the provided box. Sodium stearate (C17H35COONa) is a major component of bar soap. The Ka of the stearic acid is 1.3 × 10−5. What is the pH of 10.0 mL of a solution containing 3.96 g of sodium stearate?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

You know the right answer?
Enter your answer in the provided box. Sodium stearate (C17H35COONa) is a major component of bar soa...
Questions

Mathematics, 27.04.2021 05:20

Mathematics, 27.04.2021 05:20

Mathematics, 27.04.2021 05:20

Mathematics, 27.04.2021 05:20

Mathematics, 27.04.2021 05:20


Chemistry, 27.04.2021 05:20

Mathematics, 27.04.2021 05:20

Mathematics, 27.04.2021 05:20




Mathematics, 27.04.2021 05:20



English, 27.04.2021 05:20



Mathematics, 27.04.2021 05:20

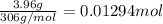
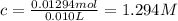
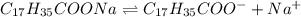
![[C_{17}H_{35}COO^-]=c=1.294 M](/tpl/images/0525/9707/45651.png)
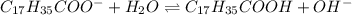
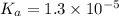
![K_a=\frac{[C_{17}H_{35}COOH][OH^-]}{[C_{17}H_{35}COO^-]}](/tpl/images/0525/9707/6f054.png)
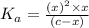
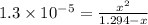
![[OH^-]=0.0041 M](/tpl/images/0525/9707/2d6f6.png)
![pOH=-\log[OH^-]=-\log[0.0041 M]=2.39](/tpl/images/0525/9707/63803.png)


