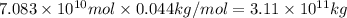Although coal is a complex mixture of substances, its elemental composition can be approximated by the formula . Using this formula, predict the amount of CO₂ released from the combustion of 1.00 × 10⁶ metric tons of coal (about the annual average for a coal-fired power plant). 1 metric ton = 1 ×10³ kg.
a. 3.66 × 10⁹ kg
b. 3.11 × 10⁹ kg
c. 8.50 × 10⁸ kg
d. 3.11 × 10⁵ kg

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3


Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Although coal is a complex mixture of substances, its elemental composition can be approximated by t...
Questions

Mathematics, 20.01.2021 22:00







Mathematics, 20.01.2021 22:00

English, 20.01.2021 22:00


English, 20.01.2021 22:00

Physics, 20.01.2021 22:00

History, 20.01.2021 22:00


Mathematics, 20.01.2021 22:00

Mathematics, 20.01.2021 22:00

Mathematics, 20.01.2021 22:00


Mathematics, 20.01.2021 22:00

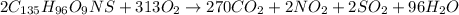
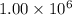 metric ton = 1.00\times 10^6\times 10^3 kg=10^9 kg[/tex]
metric ton = 1.00\times 10^6\times 10^3 kg=10^9 kg[/tex]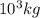
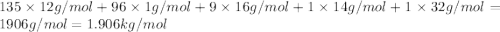
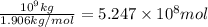
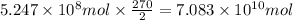 of carbon dioxide.
of carbon dioxide.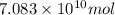 of carbon dioxide:
of carbon dioxide: