
Consider the equilibrium reaction. 2 A + B − ⇀ ↽ − 4 C After multiplying the reaction by a factor of 2, what is the new equilibrium equation? equilibrium equation: 4A + 2B<=> 8C 4 A + 2 B − ⇀ ↽ − 8 C Create the equilibrium‑constant, K c , expression for the new equilibrium reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Consider the equilibrium reaction. 2 A + B − ⇀ ↽ − 4 C After multiplying the reaction by a factor of...
Questions



Geography, 13.09.2021 01:10

Social Studies, 13.09.2021 01:10

Biology, 13.09.2021 01:10







Mathematics, 13.09.2021 01:10


Advanced Placement (AP), 13.09.2021 01:10

Physics, 13.09.2021 01:10


Mathematics, 13.09.2021 01:10


Mathematics, 13.09.2021 01:10

![K_c=\frac{[C]^8}{[A]^4[B]^2}](/tpl/images/0525/9128/9a703.png)
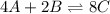
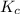 will be,
will be,

