
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented in the equation below. Ca(NO3)2(s) + 2NH4Cl(s) → 2N2O(g) + CaCl2(s) + 4H2O(g) What is the maximum volume of N2O at STP that could be produced using a 5.20-mol sample of each reactant?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2

Chemistry, 23.06.2019 08:40
A20 liter cylinder of helium at a pressure of 150 atm and a temperature of 27°c is used to fill a balloon at 1.00 atm and 37°c. what is the volume of the balloon? a. 0.14 liters b. 3000 liters c. 2900 liters d. 2400 liters e. 3100 liters
Answers: 1
You know the right answer?
Calcium nitrate will react with ammonium chloride at slightly elevated temperatures, as represented...
Questions


Law, 25.09.2021 18:10

English, 25.09.2021 18:10

Mathematics, 25.09.2021 18:10

Mathematics, 25.09.2021 18:10






Mathematics, 25.09.2021 18:10


Computers and Technology, 25.09.2021 18:10

Mathematics, 25.09.2021 18:10

Physics, 25.09.2021 18:10

Mathematics, 25.09.2021 18:10

Law, 25.09.2021 18:10

Mathematics, 25.09.2021 18:10

Social Studies, 25.09.2021 18:10

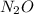 at STP produced is, 116.48 L
at STP produced is, 116.48 L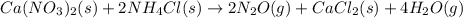
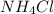 react with 1 mole of
react with 1 mole of 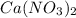
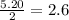 moles of
moles of 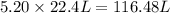 volume of gas
volume of gas 

