Chemistry, 26.02.2020 23:22 pennygillbert
Calculate the wavelength of the photon emitted when an electron makes a transition from n=6 to n=3. You can make use of the following constants: h=6.626×10−34 J⋅s c=2.998×108 m/s 1 m=109 nm

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
Calculate the wavelength of the photon emitted when an electron makes a transition from n=6 to n=3....
Questions


Business, 01.08.2019 08:00




Mathematics, 01.08.2019 08:10


Mathematics, 01.08.2019 08:10

English, 01.08.2019 08:10





Mathematics, 01.08.2019 08:10

Mathematics, 01.08.2019 08:10



Mathematics, 01.08.2019 08:10


Mathematics, 01.08.2019 08:10

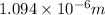
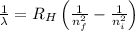
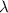 = Wavelength of radiation
= Wavelength of radiation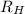 = Rydberg's Constant =
= Rydberg's Constant = 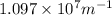
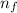 = Final energy level = 3
= Final energy level = 3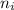 = Initial energy level = 6
= Initial energy level = 6