

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1


Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
You know the right answer?
The rate constant of a particular first order reaction is 5.45 x 10^-2 sec^-1 at 40.0 oC. What is th...
Questions


Business, 31.07.2019 13:00

Biology, 31.07.2019 13:00

Biology, 31.07.2019 13:00

Business, 31.07.2019 13:00



Social Studies, 31.07.2019 13:00

History, 31.07.2019 13:00



Biology, 31.07.2019 13:00









![\ln(\frac{K_{65^oC}}{K_{40^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0525/7564/05a35.png)
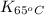 = equilibrium constant at 65°C = ?
= equilibrium constant at 65°C = ?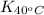 = equilibrium constant at 40°C =
= equilibrium constant at 40°C = 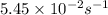
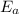 = Activation energy of the reaction = 65.5 kJ/mol = 65500 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy of the reaction = 65.5 kJ/mol = 65500 J/mol (Conversion factor: 1 kJ = 1000 J)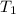 = initial temperature =
= initial temperature = ![40^oC=[40+273]K=313K](/tpl/images/0525/7564/84088.png)
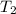 = final temperature =
= final temperature = ![65^oC=[65+273]K=338K](/tpl/images/0525/7564/eb1a6.png)
![\ln(\frac{K_{65^oC}}{5.45\times 10^{-2}})=\frac{65500J/mol}{8.314J/mol.K}[\frac{1}{313}-\frac{1}{338}]\\\\K_{65^oC}=0.350s^{-1}](/tpl/images/0525/7564/e250a.png)


