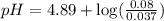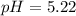Chemistry, 26.02.2020 23:18 poreally6456
A volume of 500.0 mL of 0.160 M NaOH is added to 585 mL of 0.200 M weak acid ( K a = 1.28 × 10 − 5 ) . What is the pH of the resulting buffer? HA ( aq ) + OH − ( aq ) ⟶ H 2 O ( l ) + A − ( aq

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

You know the right answer?
A volume of 500.0 mL of 0.160 M NaOH is added to 585 mL of 0.200 M weak acid ( K a = 1.28 × 10 − 5 )...
Questions

History, 16.01.2020 06:31

Mathematics, 16.01.2020 06:31

Mathematics, 16.01.2020 06:31

Mathematics, 16.01.2020 06:31



Mathematics, 16.01.2020 06:31

Spanish, 16.01.2020 06:31


Business, 16.01.2020 06:31

Mathematics, 16.01.2020 06:31

Mathematics, 16.01.2020 06:31

History, 16.01.2020 06:31

English, 16.01.2020 06:31


History, 16.01.2020 06:31

Mathematics, 16.01.2020 06:31



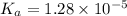
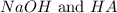
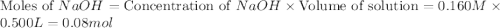
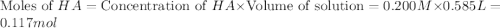
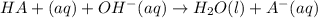
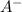 = 0.08 mol
= 0.08 mol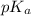 is,
is,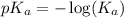
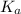 in this expression, we get:
in this expression, we get: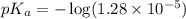
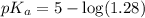
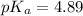
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0525/7311/e961a.png)
![pH=pK_a+\log \frac{[A^-]}{[HA]}](/tpl/images/0525/7311/a5883.png)