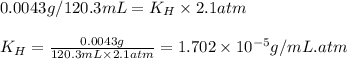Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1


Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3
You know the right answer?
If 120.3 mL of water is shaken with oxygen gas at 2.1 atm, it will dissolve 0.0043 g O2. Estimate th...
Questions



Mathematics, 20.10.2020 01:01


History, 20.10.2020 01:01

History, 20.10.2020 01:01

Physics, 20.10.2020 01:01

History, 20.10.2020 01:01

Health, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01

Chemistry, 20.10.2020 01:01

Business, 20.10.2020 01:01

History, 20.10.2020 01:01


Physics, 20.10.2020 01:01

Biology, 20.10.2020 01:01

History, 20.10.2020 01:01


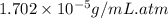
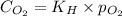
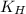 = Henry's constant = ?
= Henry's constant = ?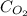 = solubility of oxygen gas =
= solubility of oxygen gas = 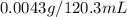
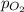 = partial pressure of oxygen gas = 2.1 atm
= partial pressure of oxygen gas = 2.1 atm