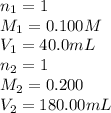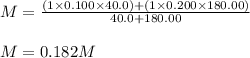Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
A solution was prepared by mixing 40.00 mL of 0.100 M HNO3 and 180.00 mL of 0.200 M HNO3 . Calculate...
Questions

Mathematics, 29.04.2021 22:50



Chemistry, 29.04.2021 22:50


Mathematics, 29.04.2021 22:50


History, 29.04.2021 22:50

Biology, 29.04.2021 22:50

Biology, 29.04.2021 22:50


Mathematics, 29.04.2021 22:50

Mathematics, 29.04.2021 22:50






Mathematics, 29.04.2021 22:50

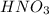 in the final solution is 0.182 M
in the final solution is 0.182 M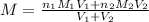
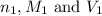 are the n-factor, molarity and volume of the
are the n-factor, molarity and volume of the 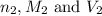 are the n-factor, molarity and volume of the
are the n-factor, molarity and volume of the