
Chemistry, 26.02.2020 21:32 ddmoorehouseov75lc
At equilibrium, the concentrations are [H2] = 5.0 M, [N2] = 10 M, and [NH3] = 3.0 M. What were the concentrations of nitrogen gas and hydrogen gas that were reacted initially?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1


Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
At equilibrium, the concentrations are [H2] = 5.0 M, [N2] = 10 M, and [NH3] = 3.0 M. What were the c...
Questions




History, 01.07.2020 22:01





Computers and Technology, 01.07.2020 22:01



Mathematics, 01.07.2020 22:01

Mathematics, 01.07.2020 22:01







![[H_2]_0=0.5M](/tpl/images/0525/5015/df212.png)
![[N_2]_0=8.5M](/tpl/images/0525/5015/80658.png)
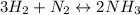
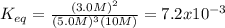
![\ \ \ \ \ \ \ \ \ \ \ \ 3H_2\ \ \ +\ \ \ \ N_2\ \ \ \ \leftrightarrow \ \ \ \ 2NH_3\\I\ \ \ \ \ \ \ \ \ \ \ [H_2]_0\ \ \ \ \ \ \ [N_2]_0\ \ \ \ \ \ \ \ \ \ \ \ \ \ 0\\C\ \ \ \ \ \ \ \ \ \ \ -3x\ \ \ \ \ \ \ \ \ \ x\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ 2x\\E\ \ \ \ \ \ \ \ [H_2]_0-3x\ \ [N_2]_0-x\ \ \ \ \ \ \ \ \ 2x](/tpl/images/0525/5015/9f9db.png)
 " due to the reaction is computed via the equilibrium concentration of ammonia as shown below:
" due to the reaction is computed via the equilibrium concentration of ammonia as shown below: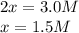
![[H_2]_0=5.0M-3(1.5M)=0.5M](/tpl/images/0525/5015/60025.png)
![[N_2]_0=10.0M-1.5M=8.5M](/tpl/images/0525/5015/9d364.png)



