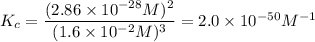Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript...

Chemistry, 26.02.2020 20:55 jessicajamah3289
Consider the reaction.
3 upper O subscript 2 (g) double-headed arrow 2 upper O subscript 3 (g).
At 298 K, the equilibrium concentration of O2 is 1.6 x 10-2 M, and the equilibrium concentration of O3 is 2.86 x 10-28 M. What is the equilibrium constant of the reaction at this temperature?
A) 2.0 x 10^-10
B) 2.0 x 10^10
C) 1.8 x 10^-10
D) 1.8 x 10^10

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2



Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
Questions




Geography, 09.03.2021 05:10






Mathematics, 09.03.2021 05:10

Arts, 09.03.2021 05:10

Mathematics, 09.03.2021 05:10


History, 09.03.2021 05:10


Mathematics, 09.03.2021 05:10


Mathematics, 09.03.2021 05:10


History, 09.03.2021 05:10


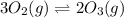
![K_c=\dfrac{[O_3g)]^2}{[O_2(g)]^3}](/tpl/images/0525/4129/a4203.png)