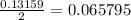Chemistry, 26.02.2020 19:29 whitneyt3218
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for each of the following initial amounts of reactants. Part A 5.0 g Ti, 5.0 g F2 Express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for ea...
Questions



History, 07.11.2020 02:30

History, 07.11.2020 02:30

History, 07.11.2020 02:30

Mathematics, 07.11.2020 02:30



History, 07.11.2020 02:30



Mathematics, 07.11.2020 02:30



Mathematics, 07.11.2020 02:30

History, 07.11.2020 02:30


Mathematics, 07.11.2020 02:30


Mathematics, 07.11.2020 02:30


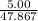
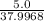
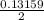 mole of Ti will react with the 0.13159 mole of F₂
mole of Ti will react with the 0.13159 mole of F₂