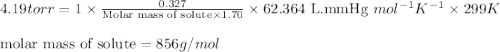A saturated solution is made by dissolving 0.327 g of a polypeptide (a substance formed by joining together in a chainlike fashion some number of amino acids) in water to give 1.70 L of solution. The solution has an osmotic pressure of 4.19 torr at 26 °C. What is the approximate molecular mass of the polypeptide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

You know the right answer?
A saturated solution is made by dissolving 0.327 g of a polypeptide (a substance formed by joining t...
Questions

Biology, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20



History, 09.04.2021 19:20




Arts, 09.04.2021 19:20



Mathematics, 09.04.2021 19:20


Arts, 09.04.2021 19:20



History, 09.04.2021 19:20

Mathematics, 09.04.2021 19:20

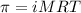
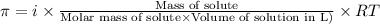
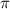 = osmotic pressure of the solution = 4.19 torr
= osmotic pressure of the solution = 4.19 torr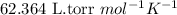
![26^oC=[273+26]K=299K](/tpl/images/0525/2386/ee574.png)