
Chemistry, 26.02.2020 19:33 ondreduty1789
For the elementary reaction NO3 + CO → NO2 + CO2 the molecularity of the reaction is , and the rate law is rate = . For the elementary reaction NO3 + CO NO2 + CO2 the molecularity of the reaction is , and the rate law is rate = . 2, k[NO3][CO]/[NO2][CO2] 4, k[NO3][CO][NO2][CO2] 2, k[NO2][CO2] 4, k[NO2][CO2]/[NO3][CO] 2, k[NO3][CO]

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
For the elementary reaction NO3 + CO → NO2 + CO2 the molecularity of the reaction is , and the rate...
Questions

Mathematics, 25.03.2020 23:58

History, 25.03.2020 23:59

Mathematics, 25.03.2020 23:59

Mathematics, 25.03.2020 23:59


Mathematics, 25.03.2020 23:59

Engineering, 25.03.2020 23:59

Advanced Placement (AP), 25.03.2020 23:59


English, 25.03.2020 23:59




Mathematics, 25.03.2020 23:59


Mathematics, 25.03.2020 23:59

Mathematics, 25.03.2020 23:59


Mathematics, 25.03.2020 23:59

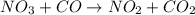 the molecularity of the reaction is 2, and the rate law is rate =
the molecularity of the reaction is 2, and the rate law is rate = ![k[NO_3]^1[CO]^1](/tpl/images/0525/2395/d7c50.png)
![rate=k[NO_3]^1[CO]^1](/tpl/images/0525/2395/3c278.png)


